Every aspect of our work and the decisions we make are guided by our research - it’s at the centre of everything we do. The science behind this is both fascinating and complex, so we’ve answered some of the most important questions about our work in four key areas of interest.
Stem cell transplants
Finding a suitable donor
Joining the stem cell register
Cell and gene therapies
.
Stem cell transplants
- What are stem cells?
- Why do some people need a stem cell transplant?
- How does a stem cell transplant work?
What are stem cells?
Stem cells are unique cells of the body that can divide to make more copies of themselves and are able to give rise to specialised cells such as white blood cells. All other cells, tissue, organs and bones develop from stem cells. Usually, the stem cells found in bone marrow produce exactly the right amount of each type of blood cell – red blood cells, platelets and white blood cells - to keep us healthy.
Why do some people need a stem cell transplant?
The development of the blood (or hematopoietic) system is a carefully controlled process but when things go wrong it can cause our body to produce too many blood cells that are not fully developed, so they can’t do their job properly. This can potentially develop into blood cancers or blood disorders.
Most of the time, we have no control over when or how these problems might develop. However, there are certain lifestyle choices, such as smoking, or genetic factors that can increase the risk of us developing cancer at some point in our lifetime.
How does a stem cell transplant work?
Before a stem cell transplant can take place, a patient will undergo conditioning therapy to remove their own stem cells and any abnormal cells causing their condition. This consists of chemotherapy and sometimes radiotherapy as well.
The donor’s stem cells are then used to replace the patient’s stem cells. The transplant itself is very similar to a blood transfusion but takes a little longer. Afterwards, the stem cells travel in the blood of the patient to the bone marrow where they attach (called engraftment) and grow. They then start to make new blood cells including white blood cells that form a new immune system.
Finding a suitable donor
- How do we match donors to people in need of a stem cell transplant?
- What does a good match look like?
- Why are HLA genes so important?
- How do we get our HLA genes?
- What happens if there is no match in the family?
- How hard is it to find a genetic match?
- Why is it harder for patients from minority ethnic backgrounds to find a matching donor?
- Why is the donor’s age important?
- Is anything else important?
How do we match donors to people in need of a stem cell transplant?
For a transplant to take place, we need to find a donor whose tissue type closely matches the patient’s. Matching is based on our human leukocyte antigen (HLA) tissue type. Our HLA is part of what makes us all unique – it’s part of our individual genetic characteristics.
There are many HLA genes but when it comes to matching, we are most interested in six of them. Each of us has two different versions (called alleles) of these genes, making 12 in total.
What does a good match look like?
If we find a donor where all twelve of these alleles match up it’s called a 12/12 match. If there’s one mismatch it’s an 11/12 match. It’s important that we find the closest match because it will give the best possible chance of the patient’s body accepting the donated cells and reduce the likelihood of post-transplant complications. We also consider a few other factors when considering a match, which you can read more about below.
Did you know?
The World Health Organisation (WHO) only recognises new HLA DNA sequences after they have been accepted into the IPD-IMGT/HLA database curated by Anthony Nolan.
Why are our HLA genes so important?
The proteins made by our HLA genes help create a barcode of the proteins found within our cells. They display this barcode on the outside of each cell so they can be inspected by cells of the immune system. White blood cells, such as T-cells, can scan this barcode and use it to decide if the cell is healthy and normal. If the T-cell detects an abnormal signal, which suggests the cell could be cancerous or has become infected, it will target the cell for destruction.
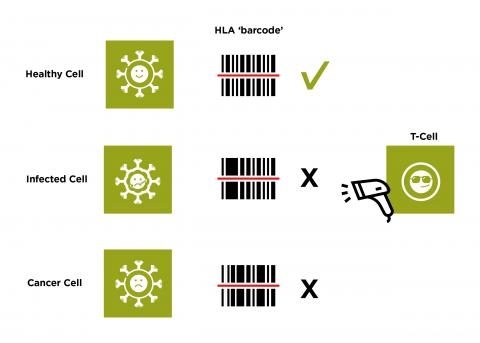
How do we get our HLA genes?
Our HLA genes are all located close to each other on chromosome six, which means they are inherited together as blocks or combinations known as haplotypes from our parents. We inherit one set from our mother and one from our father. This is why brothers or sisters are tested first because they have the best chance of being a perfect match.
There is a 1 in 4 chance (25%) of a sibling having the exact same tissue type. There is also a 2 in 4 (50%) chance of being a half match, which means they could be suitable for a haploidentical transplant which you can read more about below. Parents are also always a half match to their children and visa-versa.
Did you know?
There are more than 20,000 unique versions of HLA class I genes in our reference database and there are more variants being added all the time.
What happens if there is no match for a patient?
If a suitable related donor is not available, Anthony Nolan searches its register, and others around the world, for an unrelated donor. There are over 38 million donors on the registers worldwide and over 800,000 on the Anthony Nolan register alone.
We will also consider using donated cord blood as a source of stem cells or a haploidentical transplant. Both options have their own unique advantages.
Cord transplant – When a new mum gives birth, blood stem cells from the umbilical cord can be collected and frozen. These stem cell don’t need to be as well matched with the patient’s HLA tissue type because they are not as mature as blood stem cells. This means it can be easier to find a suitable cord match and patients are less likely to develop graft versus host disease (GvHD) afterwards too.
Read more about cord transplants.
Haploidentical transplant – These transplants use stem cells from a family member whose tissue type or HLA is a half match to the patient. Parents are always a half-match for their children, and vice versa. Siblings have a 50% chance of being a half-match for each other. Many people have at least one potential haploidentical match in their family so there is a greater choice of potential donors.
Read more about haploidentical transplants.
How hard is it to find a genetic match?
Our HLA genes are the most varied in our entire genome – with thousands and thousands of known variations. This genetic variation has evolved to combat the wide range of bacteria, viruses and fungi we come into contact with daily, without even realising. It means there is less chance of a single disease wiping out the entire human race. We simply would not be able to survive without our HLA genes.
However, this diversity does make it more difficult to find a suitable matching donor. This is why we are working hard to add more potential donors to our register to give us the best possible chance of finding a match for every patient.
Why is it harder for patients from minority ethnic backgrounds to find a matching donor?
Patients are more likely to find a matching donor from someone with a similar ethnic background because our HLA tissue types are inherited – although it’s possible to be a donor for someone of any ethic background. Genetic diversity is created when different populations mix together, which means some ethnicities have rarer HLA tissue types than others. This makes it harder for us to find a good match for some patients compared to others.
To resolve this problem, we need to work in the UK and internationally to add more people from minority ethnic backgrounds to the stem register. We are also promoting the use of cord blood as an alternative source of stem cells and researching ways to make them more accessible. All of this combined will provide a larger pool of potential donors with diverse tissue types to help all patients have an equal chance of finding a match.
Why is the donor’s age so important?
Our research shows that after HLA matching, the age of a stem cell donor is the most important characteristic that influences stem cell transplant success rates – with younger donors associated with better survival rates. Based on a study of over 1,200 transplants, Anthony Nolan became the first UK stem cell register in the world to lower the age limit for joining to 16 and introduce an upper age limit of 31. Other UK stem cell registers currently recruit up to the ages of 45 and 55.
Is anything else important?
Yes - Cytomegalovirus (or CMV for short) is a very common virus that usually has no side effects. However, it can lead to complications in patients after their stem cell transplant before their new immune system develops.
We test both donors and patients to see if they’ve had CMV in the past because evidence shows that when a patient and donor have a matched CMV status it helps improve transplant success.
We know there’s still lots to learn when it comes to what makes the perfect match. Our Patient/Donor Project, run by our immunogenetics team, brings together samples from over 1,500 transplants to determine which factors relate to positive transplant outcomes.
Did you know?
Stem cells from cord blood are not as mature as adult blood stem cells which means they don’t need to be as well matched. They are also less likely to cause complications like GvHD.
Joining the stem cell register
- What happens to the cheek swab when someone joins the register?
- How is the HLA tissue type determined?
- How likely is it that I will be asked to donate my stem cells?
What happens to the cheek swab when someone joins the register?
When someone takes a swab of the cells in their mouth for us, it’s the first step in determining their HLA tissue type so they can be added to the register. After we receive the swabs at our labs, we use a combination of in-house testing and our referral lab where we extract and purify DNA from the cells. After running some tests to check that the DNA is of high quality, it is then amplified and processed so there is enough DNA ready for sequencing.
How is the HLA tissue type determined?
The DNA from the swab is processed by the lab by targeting the areas important for our immune system. This produces millions of copies of the DNA, that is loaded onto the sequencer which is effectively an instrument full of extremely sensitive digital cameras. Each time a new individual DNA base is sequenced, one of four coloured flashes of light is given off depending on the type of base added (these are known as ‘A’, ‘T’, ‘C’ or ‘G’). The camera detects these flashes and uses them to ‘read’ the DNA sequences.
We then analyse the DNA sequence of each gene, which allows us to define which specific type (allele) of each HLA the potential donor has inherited. Once complete, the donor is then added to our register.
The whole process is completed in just four days.
How likely is it that I will be asked to donate my stem cells?
In general, roughly one in every 800 donors are selected as a lifesaving match for a patient. However, if you are a young male donor and your first swab sample shows that you have a common HLA tissue type, you could be contacted by our donor enrichment team. This is because you are more likely to be a suitable donor for one of our patients.
With your permission, they will arrange for you to have some further testing. This includes a more detailed analysis of your HLA from a blood sample and testing your CMV status. With this additional information we can be more confident that any potential transplant you donate your stem cells to, will be a success. There is a 1 in 20 chance of a donor who is part of the register enrichment programme becoming a lifesaving saving donor.
Cell and gene therapies
- What are cell and gene therapies?
- What are CAR T-cells?
- How do CAR T-cells work?
- How is Anthony Nolan helping to develop these new therapies?
What are cell and gene therapies?
- Cell therapies: One of the most promising new areas of research uses cell therapies, where certain cell types from a donor, or even a patient’s own cells, are given as a treatment. These cells act as a ‘living drug’ because we take advantage of their natural function in the body. When they are given to the patient, they either stop the problematic cells from working or remove them completely.
- Gene therapy: There are many inherited conditions that are caused by changes (mutations) in our DNA. The aim of gene therapy is to directly correct these changes by introducing the correct version of a gene or DNA sequence into the affected cells of the patient.
Although very promising, only a few gene therapies have been approved for use in patients and most treatments are still being tested and developed. However, these therapies offer the hope of a potential cure for some patients who do not have any other options available to them.
It is also possible to combine aspects of cell therapy and gene therapy into the same treatment. Living cells that have been collected from either a patient, or a willing donor, can be genetically altered in the laboratory to change or improve their natural functions. This is the basis for developing CAR T-cell therapies.
What are CAR T-cells?
T-cells are an important part of our immune system. They recognise abnormal cells, such as cancerous cells, and destroy them. They do this through receptors on their surface that bind to proteins found on cancerous cells but not on normal cells. When T-cells are collected from the blood, scientists can manipulate their DNA so that they make new proteins, including new types of receptors. These receptors are known as Chimeric Antigen Receptors (CAR), which means the T-cells become CAR T-Cells.
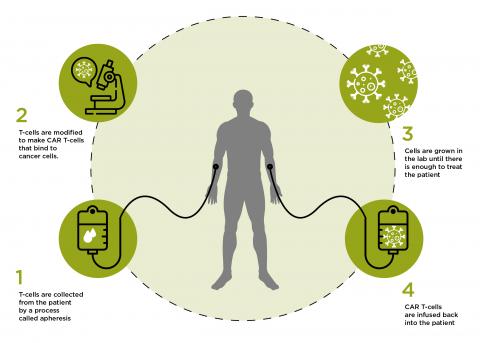
How do CAR T-cells work?
CARs have two main functions. Firstly they bind to targets on the cancer cell, but they also send out signals that attract other immune cells to the site of the cancer cell and instruct them to rapidly reproduce. This increases the chance of all cancer cells being removed.
The first CAR T-cell therapies to be approved all targeted a protein called CD19 that is found in large amounts on cells that cause acute lymphoblastic leukaemia (ALL) and some types of lymphoma. There are now hundreds of other CAR T-cell treatments being developed and tested in clinical trials around the world that target different proteins and types of cancer.
How is Anthony Nolan helping to develop these new therapies?
For over 45 years we have signed up potential stem cell donors to our register – people who are willing to donate their cells to someone in need of a lifesaving stem cell transplant. As this work continues, in parallel we are applying our vast experience and dedicated team of experts to help develop new cell-based therapies.
Our Cell and Gene Therapy Services were created to provide blood and stem cells for ethically approved research projects. It involves our donors who have expressed an interest in donating their cells for research. The organisations we are working with are transforming the cell and gene therapy space by developing new treatments that will hopefully save and improve the lives of patients around the world.
Our immunotherapy research group uses blood cells donated for research to develop new and exciting cell therapies to treat a range of post-transplant complications.